Section 3: Calculating the Volume
If you know the molarity and the number of moles of a component present, you can calculate the volume of the total system.
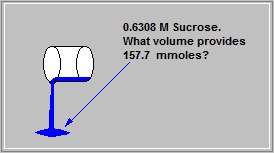
What volume in ml of a 0.6308 M Sucrose solution contains 157.7 millimoles of Sucrose?
Click to Remove Question
250 ml of solution contains 157.7 millimoles of Sucrose.
Volume of Total System (in dm3 ) = Number of Moles
Molarity
157.7 millimoles = 0.1577 moles
Thus: Volume of Sucrose Solution = 0.1577 dm3
0.6308
Volume of Sucrose Solution = 0.25 dm3
Volume of Sucrose Solution = 1000 x 0.25 ml = 250 ml
Molarity = Number of Moles
Volume of Total System (in dm3 )
which rearranges to:Volume of Total System (in dm3 )
Volume of Total System (in dm3 ) = Number of Moles
Molarity
157.7 millimoles = 0.1577 moles
Thus: Volume of Sucrose Solution = 0.1577 dm3
0.6308
Volume of Sucrose Solution = 0.25 dm3
Volume of Sucrose Solution = 1000 x 0.25 ml = 250 ml
Click to Continue