Introduction to "parts" Terminology
The approximate solubility of a substance can be defined using the expression "PARTS".
For example:
"Boric Acid is soluble in 20 parts of water"
What does this expression actually mean?

Click to Continue
Solubility
Under a set of conditions, there is a maximum amount of solute that can dissolve in a solvent. When the maximum amount of solute has dissolved, the solution is saturated and has reached the maximum concentration of that solute in the solvent for the current set of conditions.
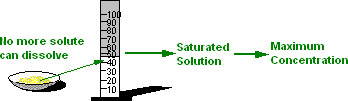
This maximum concentration is the SOLUBILITY of that solute in the solvent.
Solubility is often expressed in "parts" terminology eg
"Aspirin is soluble in 25 parts ethanol."
"Aspirin is soluble in 25 parts ethanol."
Click to Continue