Solubility Parts Expressions
How can solubility expressions be used to find
the concentration of solutions?
Solubility expressions indicate the MINIMUM VOLUME of a solvent needed to dissolve 1 gram (or 1 millilitre) of a solute, under a particular set of conditions.
Solubility expressions therefore tell you:
- 1. the concentration of a SATURATED solution of a solute
- 2. the MAXIMUM concentration possible for that solute
Boric Acid is soluble in 20 parts of water.
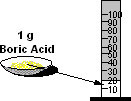 This means that:
This means that:
1 gram of Boric Acid dissolves in 20 millilitres of water.
You can therefore find how many MOLES of
Boric Acid there are in 20 millilitres of water. Moles of Boric Acid = Mass/RMM = 1 / 61.8 = 0.01618 moles
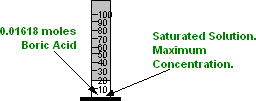
Thus, a SATURATED solution of Boric Acid contains
0.01618 moles of Boric Acid for every 20 millilitres of water.
0.01618 moles of Boric Acid for every 20 millilitres of water.
And, the MAXIMUM concentration of Boric Acid possible is:
0.01618 moles of Boric Acid in every 20 millilitres of water.
0.01618 moles of Boric Acid in every 20 millilitres of water.
Click to Remove Question