Understanding Dissolution
Dissolution rate may be defined as the amount of active ingredient in a solid-dosage form dissolved in unit time under standardized conditions of liquid-solid interface, temperature, and media composition.
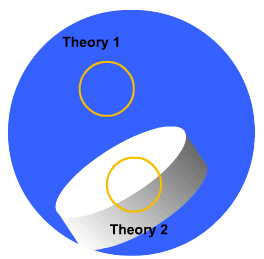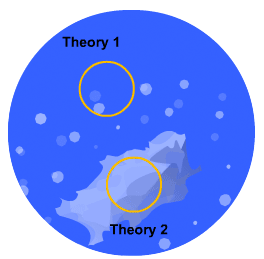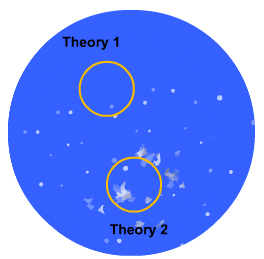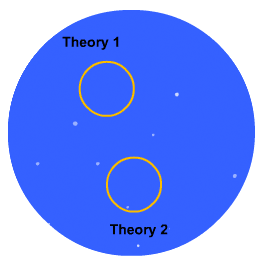
There are two different dissolution rate theories that attempt to describe the events that take place at the particle - solvent interface. They are known as the Diffusion Layer Model (Theory 1) and the Surface Renewal Theory (Theory 2) respectively.
Solid dosage form dissolving
(Tablet shown here for example)
Active Pharmaceutical Ingredient (API)
Solvent Bulk Solution Concentration
Click on the blue text across or on the orange highlighting circles below to view the dissolution theories.
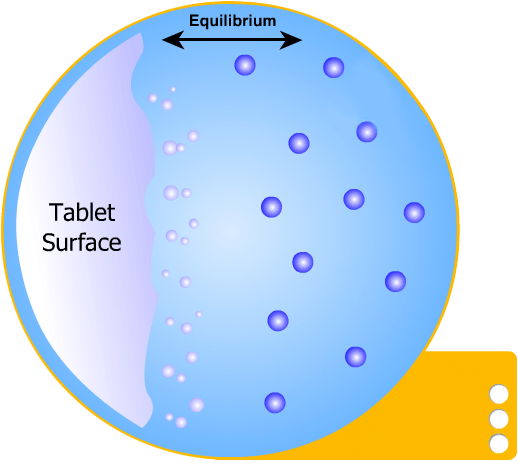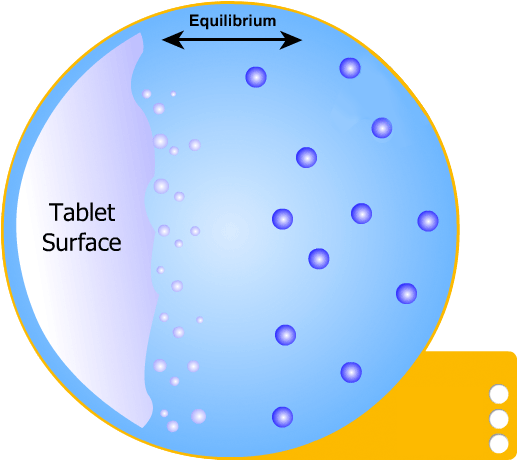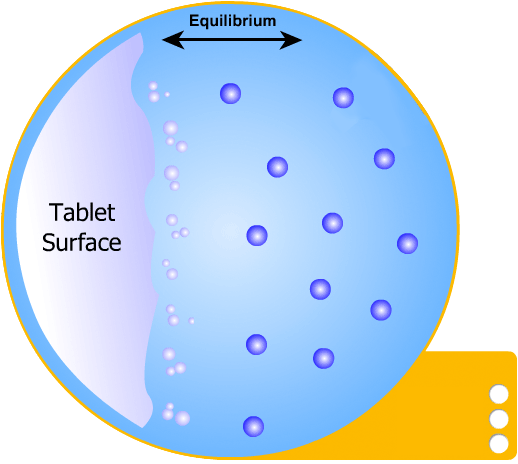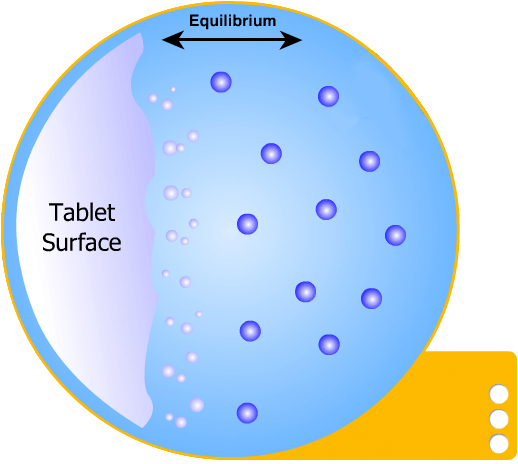
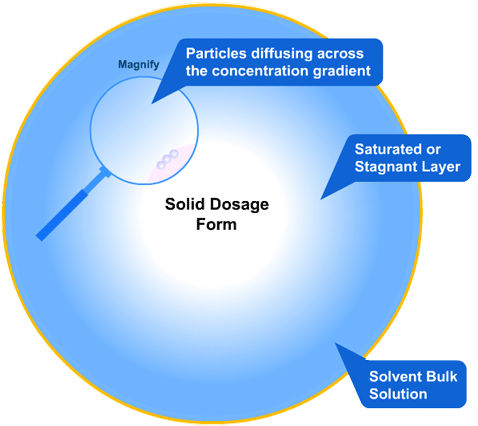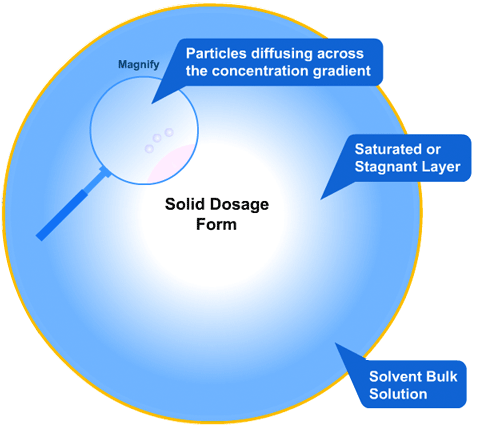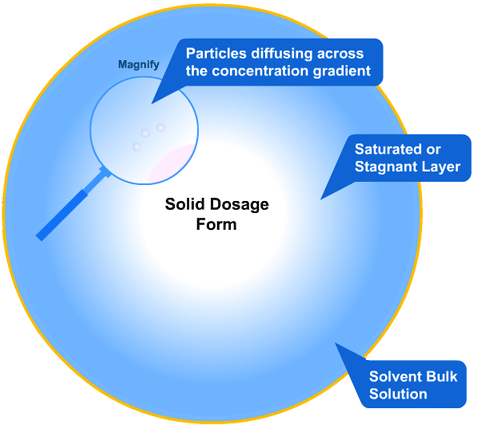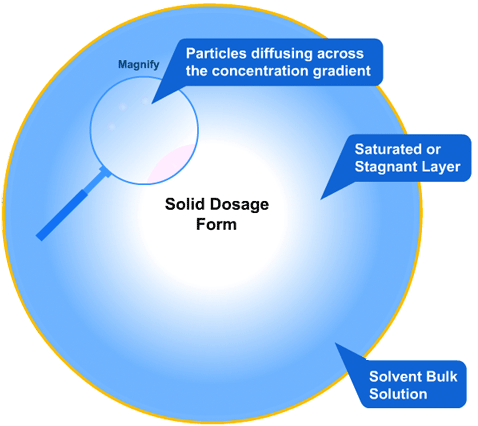
This is the simplest of the two models to understand: the solid tablet begins to release particles into solution until the region immediately surrounding the tablet becomes saturated. When this happens, no more particles can dissolve.
However, a concentration gradient now exists from the concentrated saturated layer to the bulk solution - particles diffuse across this gradient, maintaining saturated layer.
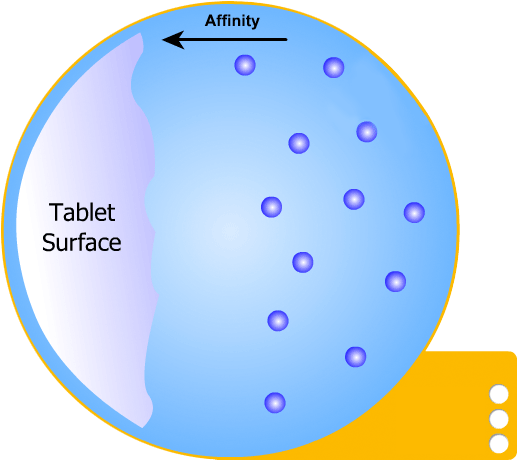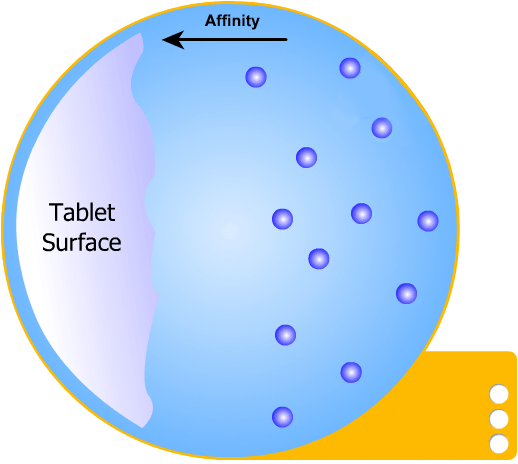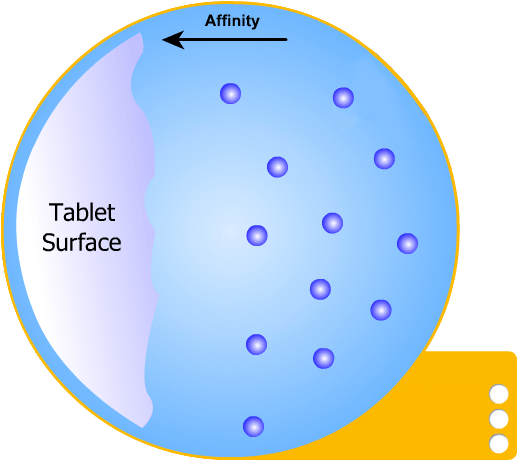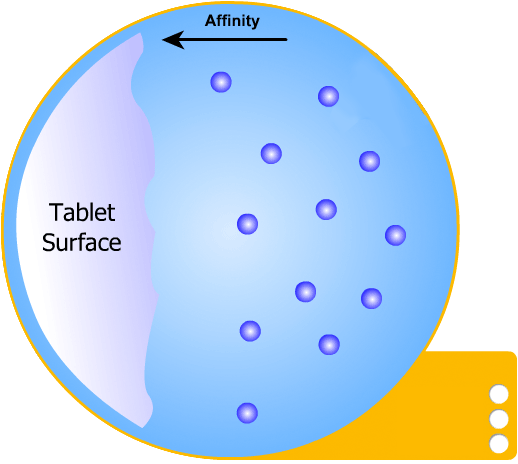
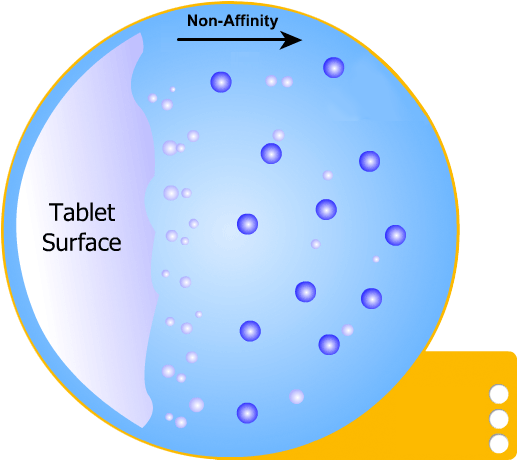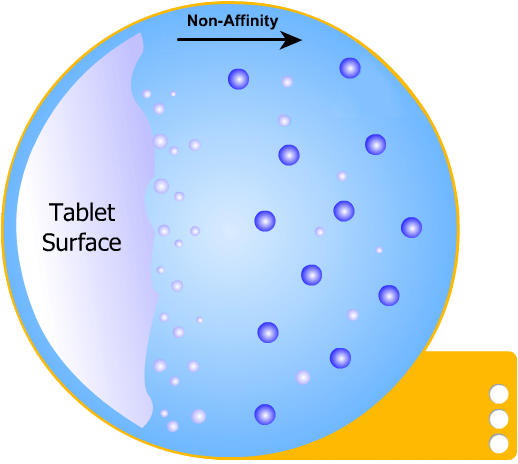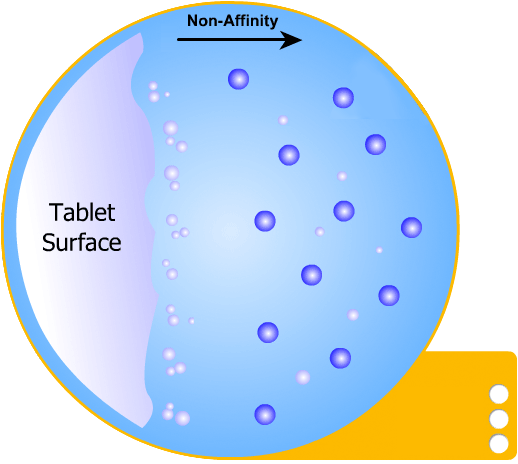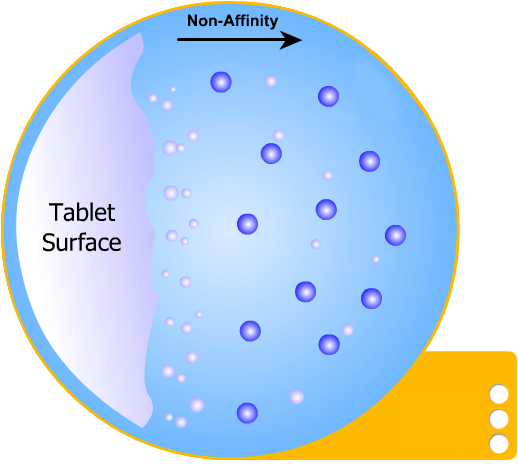
In this more complicated theory, the solvent (blue) initially gains an affinity for the tablet surface and encourages it to dissolve, causing the region around the tablet surface to become saturated. Free tablet particles (purple) now develop a non-affinity with other particles in this layer and are encouraged to pass into the bulk solution. This situation exists in a state of dynamic equilibrium.