Performing the Dissolution Test
1 Clean
Equipment2 Prepare and
Measure Media3 Add Media
to Vessel4 Adjust Media
Temperature5 Examine
Dosage Units6 Choose
Dosage Units7 Record
Information8 Prepare to
Drop Tablets9 Drop
Tablets10 Withdraw
Sample11 Filtrate to
Stop Test12 Determine
Concentration
Verify that the Equipment is Clean
VERIFY
The analyst is responsible for verification of the physical parameters.

Prepare and Measure Dissolution Media
USP PREPARATION METHOD
- Heat media to 41°C
- Vacuum filter through 0.45µm filter
- Continue to pull vacuum for five additional minutes




MEASURING MEDIA
Introduce Dissolution Media to the Vessel
PREPARE
Media addition has to be done carefully and accurately. Have materials ready for the test, including all sampling equipment.

Adjust the Media Temperature in each of the Vessels
TEMPERATURE
Allow media in each vessel to reach 37 °C ± 0.5 °C and use immediately.

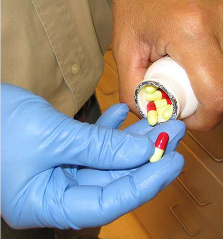
Examine the Six Dosage Units
EXAMINATION
Do not use chipped, cracked or capped
tablets.
Always handle dosage units with gloves (not cotton), forceps or tweezers which will not scratch or damage the surface of the dosage unit.
Record the Dosage Unit Weight
THIS IS AN OPTIONAL STEP
Weight is for information and investigation purposes only. Dosage units must be chosen at random and may not be selected or discarded based on weight.

Record the Information Immediately
DOCUMENTATION
An analyst notebook or an official batch record worksheet must be made available to directly record information during the test.

Prepare to Drop the Tablets
PREPARATION
Lower paddles to proper height, or suspend baskets until ready to begin the test. Apparatus 1 baskets should be tested immediately after placing the tablet or capsule inside and clipping to the shaft. Exposure to humidity can alter test results.

CALIBRATED TIMER
Prepare a calibrated timer to record times when tablets are dropped.

Drop the Tablets
APPARATUS 2
For the paddle apparatus, drop the dosage units into non-rotating medium. They must settle to the bottom of the vessel before rotation of the shaft begins.

IMPORTANT
Visually inspect the dosage forms for air bubbles immediately after dropping. Record any unusual observations.
Withdraw the Sample at the Proper Time
SAMPLING ACCURACY
Withdraw sample at the proper time ± 2% (eg a 30 minute sample must be pulled within ± 36 seconds) and filter immediately. Temperature must be taken a second time at least, generally after the last sample is pulled and before the shaft has stopped.

Filtrate to Stop Test
FILTRATION
Filtration stops the dissolution process and defines the end of the first phase of the test which is basically a sample preparation period executed under strictly controlled physical parameters. Once the sample has been filtered the second phase of the testing begins to determine the analytical concentration of the sample.
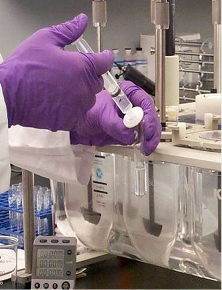
Determine Concentration
THE ANALYTICAL FINISH
Analytical concentration is generally determined through UV-Vis spectroscopy or HPLC analysis. HPLC is primarily used for drug products containing multiple active components. The responses from the analytical finish will be used to calculate the amount of sample released from the dosage form within the specific time interval.

