Introduction To Agonists: Theory
Equation For Occupancy
So, you have SEEN how occupancy varies with the agonist concentration but what is the equation that describes this variation?
The equation is found from looking at the reaction between the agonist and receptor molecules and applying the Law of Mass Action.
The law of mass action says that the RATE of a reversible chemical reaction is proportional to the PRODUCT of the concentration of the REACTANTS. So for the following reaction:
A + B 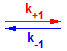 C + D
C + D
 C + D
C + Dk+1 = Forward Rate Constant k-1 = Backward Rate Constant